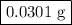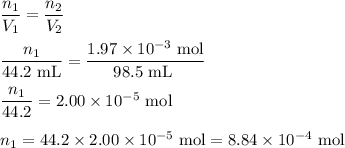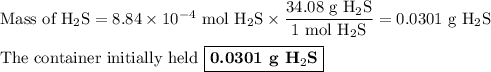Chemistry, 28.06.2019 05:50 dinarussell74
Hydrogen sulfide gas (h2s) is a highly toxic gas that is responsible for the smell of rotten eggs. the volume of a container of hydrogen sulfide is 44.2ml. after the addition of more hydrogen sulfide, the volume increases to 98.5ml under constant pressure and temperature. the container now holds 1.97×10−3mol of the gas. how many grams of hydrogen sulfide were in the container initially? give your answer in three significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Hydrogen sulfide gas (h2s) is a highly toxic gas that is responsible for the smell of rotten eggs. t...
Questions

Biology, 14.01.2021 01:00

SAT, 14.01.2021 01:00



English, 14.01.2021 01:00





Mathematics, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

English, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00