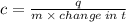When 45 g of an alloy, at 25°c, are dropped into 100.0g of water, the alloy absorbs 956j of heat. if the temperature of the alloy is 37°c, what is its specific heat? a. 0.423 cal/g°c b. 1.77 cal/g°c c. 9.88 cal/g°c d. 48.8 cal/g°c try and explain with step by step or show work,

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
When 45 g of an alloy, at 25°c, are dropped into 100.0g of water, the alloy absorbs 956j of heat. if...
Questions


Mathematics, 25.08.2021 03:20


Mathematics, 25.08.2021 03:20



Mathematics, 25.08.2021 03:20


Mathematics, 25.08.2021 03:20


Social Studies, 25.08.2021 03:20



Mathematics, 25.08.2021 03:20

Mathematics, 25.08.2021 03:20



Advanced Placement (AP), 25.08.2021 03:20

Mathematics, 25.08.2021 03:20

Mathematics, 25.08.2021 03:20