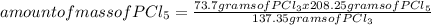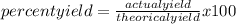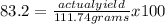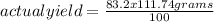Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2


Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
The percentage yield for the reaction pcl3 + cl2 → pcl5 is 83.2%. what mass of pcl5 is expected fro...
Questions

Social Studies, 15.10.2019 03:10








Mathematics, 15.10.2019 03:10




Chemistry, 15.10.2019 03:10

Computers and Technology, 15.10.2019 03:10

Computers and Technology, 15.10.2019 03:10