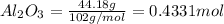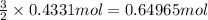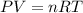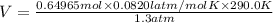Chemistry, 29.06.2019 10:20 gonzalesrosalinda66
During a laboratory experiment, 44.18 grams of al2o3 was formed when o2 reacted with aluminum metal at 290.0 k and 1.3 atm. what was the volume of o2 used during the experiment? 3o2 + 4al → 2al2o3 a. 10.10 liters b. 11.90 liters c. 12.51 liters d. 15.55 liters

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
During a laboratory experiment, 44.18 grams of al2o3 was formed when o2 reacted with aluminum metal...
Questions







Mathematics, 06.05.2020 06:34


Computers and Technology, 06.05.2020 06:34

Social Studies, 06.05.2020 06:34

Mathematics, 06.05.2020 06:34

English, 06.05.2020 06:34


English, 06.05.2020 06:34



Mathematics, 06.05.2020 06:34

Mathematics, 06.05.2020 06:34


Spanish, 06.05.2020 06:34

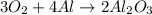
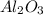 = 44.18 g
= 44.18 g