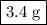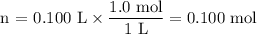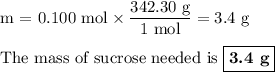Chemistry, 29.06.2019 19:10 amayaroberson3476
Using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml from c12h22o11

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100...
Questions

History, 23.04.2021 06:10


Chemistry, 23.04.2021 06:10



Biology, 23.04.2021 06:10

Mathematics, 23.04.2021 06:10


Mathematics, 23.04.2021 06:10


Chemistry, 23.04.2021 06:10







Mathematics, 23.04.2021 06:10

Mathematics, 23.04.2021 06:10

Advanced Placement (AP), 23.04.2021 06:10