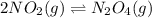Chemistry, 29.06.2019 21:50 dooboose15
In the reversible reaction: 2no2 (g) ⇌ n2o4 (g) the formation of dinitrogen tetroxide releases heat and the formation of nitrogen dioxide absorbs heat. if the reaction is at equilibrium and the temperature increases, what will the effect be? a. nitrogen dioxide absorbs heat and changes from gas to liquid. b. the equilibrium will shift so that there is more nitrogen dioxide. c. the equilibrium will shift so that there is more dinitrogen tetroxide. d. dinitrogen tetroxide absorbs heat and changes from gas to liquid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3


Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
In the reversible reaction: 2no2 (g) ⇌ n2o4 (g) the formation of dinitrogen tetroxide releases heat...
Questions


Mathematics, 29.11.2020 21:30




Business, 29.11.2020 21:30











Mathematics, 29.11.2020 21:40


Mathematics, 29.11.2020 21:40

Mathematics, 29.11.2020 21:40