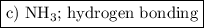Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
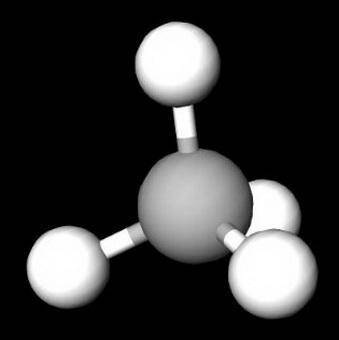
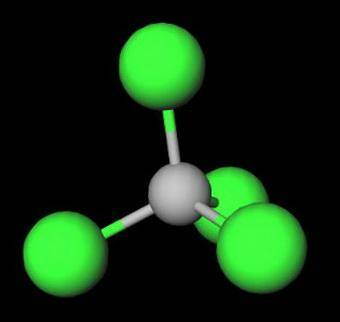
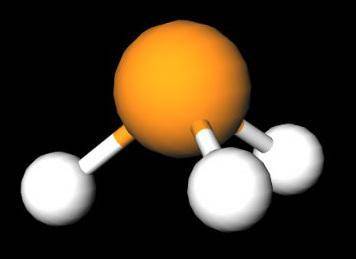
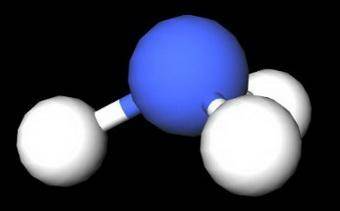
On the basis of molecular structure and bond polarity, which of the following compounds is most like...
Questions


Mathematics, 15.05.2021 01:00

World Languages, 15.05.2021 01:00

Mathematics, 15.05.2021 01:00


Mathematics, 15.05.2021 01:00

Spanish, 15.05.2021 01:00


Arts, 15.05.2021 01:00

Mathematics, 15.05.2021 01:00

Mathematics, 15.05.2021 01:00


Mathematics, 15.05.2021 01:00


Mathematics, 15.05.2021 01:00

World Languages, 15.05.2021 01:00


Mathematics, 15.05.2021 01:00


Mathematics, 15.05.2021 01:00

 .
.