

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2


Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 23.06.2019 09:30
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2
You know the right answer?
Determine the enthalpy change of the following reaction: co + h2o -> h2 + co2 given enthalpies...
Questions







Computers and Technology, 13.12.2019 07:31




Mathematics, 13.12.2019 07:31


Computers and Technology, 13.12.2019 07:31




Computers and Technology, 13.12.2019 07:31



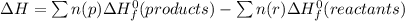
![\Delta H = [1\Delta H_{f}^{0}(H2)+1\Delta H_{f}^{0}(CO2)]-[1\Delta H_{f}^{0}(CO)+1\Delta H_{f}^{0}(H2O)]](/tpl/images/0034/1721/21403.png)


