Chemistry, 04.01.2020 14:31 battlemarshmell
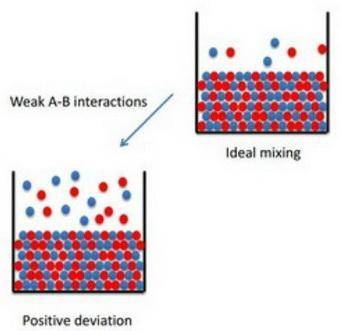
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 torr with an equimolar amount of a liquid with a vapor pressure of 200 torr. the resulting solution would be predicted to have a vapor pressure of 150 torr if it behaved ideally. if, however, the interactions between the different components are not similar we can see positive or negative deviations from the calculated vapor pressure. an actual vapor pressure greater than that predicted by raoult's law is said to be a positive deviation and an actual vapor pressure lower than that predicted by raoult's law is a negative deviation. part a imagine a solution of two liquids in which the molecules interact less favorably than they do in the individual liquids. will this solution deviate positively from, deviate negatively from, or ideally follow raoult's law? '

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:00
For a family dinner, jose’s mom baked a loaf of bread. during the meal, his grandmother commented, “this bread is so dense! ” what do you think his grandmother meant by the word dense? you have some ideas regarding density already, but this experiment will enrich your understanding of the relationship between mass, volume and the density of objects. when making measurements in this experiment we will be using si units, the international system of units. the purpose of using si units is to have the ability to communicate and compare data results with other experiments without having to make conversions. measurement symbol si unit length m meter mass kg kilogram volume l liters density kg/l kilograms per liter any calculations that are performed during this experiment will ultimately be reported in scientific notation. recall that scientific notation is extremely important when we have data that is extremely small or large. scientists rely on a standard form of communication to quickly make hypotheses and judgements based on data. what is the density of block a? a0kg/l what is the density of block b? a1kg/l what is the density of block c? a2kg/l what is the density of block d? a3kg/l what is the density of block e? a4kg/l which block is the densest? a5
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

You know the right answer?
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 to...
Questions



Physics, 31.03.2021 17:20



Biology, 31.03.2021 17:20

German, 31.03.2021 17:20

Mathematics, 31.03.2021 17:20

Mathematics, 31.03.2021 17:20

Social Studies, 31.03.2021 17:20

Social Studies, 31.03.2021 17:20


Mathematics, 31.03.2021 17:20

Social Studies, 31.03.2021 17:20



History, 31.03.2021 17:20



History, 31.03.2021 17:20