
Chemistry, 07.11.2019 06:31 YoVeoAnime
A1.50-liter sample of dry air in a cylinder exerts a pressure of 3.0 atmospheres at a temperature of 25°c. without change in temperature, a piston is moved in the cylinder until the pressure in the cylinder is reduced to 1.0 atmosphere. what is the volume of the gas? (be sure to use the correct number of significant figures.) 0.22 l 0.50 l 2.0 l 4.5 l

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

You know the right answer?
A1.50-liter sample of dry air in a cylinder exerts a pressure of 3.0 atmospheres at a temperature of...
Questions

Biology, 24.07.2019 12:50

History, 24.07.2019 12:50

Social Studies, 24.07.2019 12:50

English, 24.07.2019 12:50




Mathematics, 24.07.2019 12:50




History, 24.07.2019 12:50

History, 24.07.2019 12:50


Mathematics, 24.07.2019 12:50

History, 24.07.2019 12:50

English, 24.07.2019 12:50

Mathematics, 24.07.2019 12:50


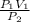
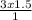 = 4.5L
= 4.5L


