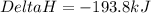Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1


Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1
You know the right answer?
What is the heat of reaction when sulfur dioxide reacts with oxygen to form sulfur trioxide? 2so2(g...
Questions

Mathematics, 10.12.2020 15:20

Mathematics, 10.12.2020 15:20

Mathematics, 10.12.2020 15:20

Mathematics, 10.12.2020 15:30

History, 10.12.2020 15:30

History, 10.12.2020 15:30



Physics, 10.12.2020 15:30


English, 10.12.2020 15:30

Mathematics, 10.12.2020 15:30

Health, 10.12.2020 15:30

Biology, 10.12.2020 15:30


Mathematics, 10.12.2020 15:30

Arts, 10.12.2020 15:30



Medicine, 10.12.2020 15:30

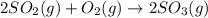
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0273/9627/76c37.png)
![\Delta H=[(n_{SO_3}\times \Delta H_{SO_3})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{SO_2}\times \Delta H_{SO_2})]](/tpl/images/0273/9627/23304.png)
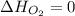 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![\Delta H=[(2\times -395.7)]-[(1\times 0)+(2\times -298.8)]](/tpl/images/0273/9627/57adc.png)