
Chemistry, 04.02.2020 09:49 greekfreekisdbz
The balanced equation for combustion in an acetylene torch is shown below:
2c2h2 + 5o2 → 4co2 + 2h2o
the acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2.
how many moles of co2 are produced when 35.0 mol c2h2 react completely?
mol co2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
The balanced equation for combustion in an acetylene torch is shown below:
2c2h2 + 5o2 → 4co...
2c2h2 + 5o2 → 4co...
Questions

English, 26.09.2019 13:30

Mathematics, 26.09.2019 13:30

Mathematics, 26.09.2019 13:30

Spanish, 26.09.2019 13:30






English, 26.09.2019 13:30



Chemistry, 26.09.2019 13:30

Mathematics, 26.09.2019 13:30


Mathematics, 26.09.2019 13:30



English, 26.09.2019 13:30

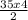 = 70mol
= 70mol

