
Chemistry, 02.02.2020 12:49 paigefields2578
Formic acid, hcooh, is a weak acid present in the venom of red-ants. at equilibrium, [hcooh] = 2.00 m, [hcoo− ] = 4.0 × 10− 1 m, and [h3o+ ] = 9.0 × 10− 4 m.
a. write the equilibrium expression for the ionization

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2


Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Formic acid, hcooh, is a weak acid present in the venom of red-ants. at equilibrium, [hcooh] = 2.00...
Questions

Mathematics, 14.02.2022 15:40


History, 14.02.2022 15:40

Mathematics, 14.02.2022 15:40


Mathematics, 14.02.2022 15:40



Computers and Technology, 14.02.2022 15:40


Mathematics, 14.02.2022 15:40


Business, 14.02.2022 15:40


Mathematics, 14.02.2022 15:40

Engineering, 14.02.2022 15:40




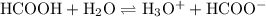
![K_{eq} = \dfrac{[\text{Products}]}{[\text{Reactants}]}](/tpl/images/0492/9894/57030.png)
![K_{eq} = \dfrac{[\text{HCOO}^{-}][\text{H}_{3}\text{O}^{+}]}{[\text{HCOOH}]}](/tpl/images/0492/9894/43500.png)


