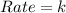Chemistry, 05.02.2020 02:50 damienlopezram
What is the overall order of reaction for this rate law: rate = k ? a. zero b. first c. second d. third

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
What is the overall order of reaction for this rate law: rate = k ? a. zero b. first c. second d....
Questions





Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01




Biology, 13.10.2020 05:01


English, 13.10.2020 05:01



Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Computers and Technology, 13.10.2020 05:01

Business, 13.10.2020 05:01

Chemistry, 13.10.2020 05:01