
Chemistry, 15.10.2019 17:30 living8539
L. given the balanced equation representing a reaction: what occurs during this reaction? 1) energy is absorbed as bonds are broken.2) energy is absorbed as bonds are formed.3) energy is released as bonds are broken4) energy is released as bonds are formed.
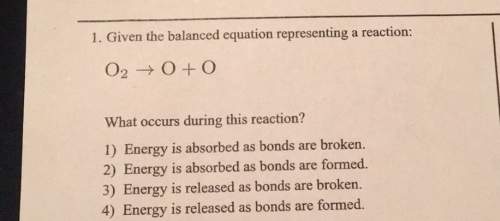

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2

Chemistry, 23.06.2019 19:00
Covalent bonds have a higher melting points than ionic bonds. true or false
Answers: 1
You know the right answer?
L. given the balanced equation representing a reaction: what occurs during this reaction? 1) energy...
Questions


Mathematics, 02.12.2020 01:00


Mathematics, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00

Social Studies, 02.12.2020 01:00

Spanish, 02.12.2020 01:00



Mathematics, 02.12.2020 01:00


Mathematics, 02.12.2020 01:00



Social Studies, 02.12.2020 01:00


Mathematics, 02.12.2020 01:00






