
Chemistry, 27.01.2020 11:31 aletadaboss
If a 25.0 ml sample of sulfuric acid is titrated with 50.0 ml of 0.200 m potassium hydroxide to a phenolphthalein endpoint, what is the molarity of the acid?
a) 0.150 m
b) 0.100 m
c) 0.200 m
d) 0.300 m
e) 0.400 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
You know the right answer?
If a 25.0 ml sample of sulfuric acid is titrated with 50.0 ml of 0.200 m potassium hydroxide to a ph...
Questions










English, 06.05.2020 00:29




Mathematics, 06.05.2020 00:29



Mathematics, 06.05.2020 00:29

Computers and Technology, 06.05.2020 00:29

Mathematics, 06.05.2020 00:29

English, 06.05.2020 00:29

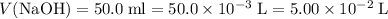 ;
;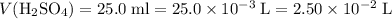 .
.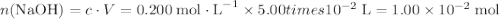 .
.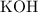 neutralizes
neutralizes 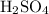 at a two-to-one ratio:
at a two-to-one ratio: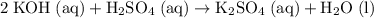 .
.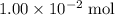 moles of
moles of 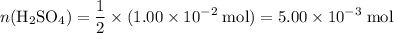 .
.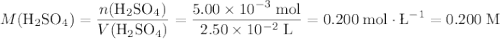 .
.

