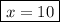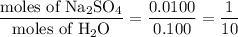Chemistry, 20.01.2020 08:31 mercedesamatap21hx0
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (molar mass 18 g) is driven off. the mass of the anhydrous na2so4 (s) (molar mass 142 g) that remains is 1.42g. the value of x in the hydrate is

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (mo...
Questions

Mathematics, 02.12.2020 01:10


Chemistry, 02.12.2020 01:10

Mathematics, 02.12.2020 01:10


Mathematics, 02.12.2020 01:10


English, 02.12.2020 01:10

Mathematics, 02.12.2020 01:10

Mathematics, 02.12.2020 01:10

Mathematics, 02.12.2020 01:10

Mathematics, 02.12.2020 01:10





Mathematics, 02.12.2020 01:10

Computers and Technology, 02.12.2020 01:10


Mathematics, 02.12.2020 01:10