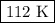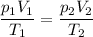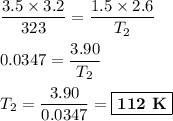Urgent 25 !
consider this gas law problem: if i have 3.2 l of gas at a pressure of 3.5 atm a...

Urgent 25 !
consider this gas law problem: if i have 3.2 l of gas at a pressure of 3.5 atm and a temperature of 323 k, what will be the temperature of the gas if i decrease the volume of the gas to 2.6 l and decrease the pressure to 1.5
answer all parts below for full credit:
a) what are the knows in this problem
b) what is the problem asking you to find
c) which gas law is the best law for finding the answer to this problem?
d) use the gas law that you indicated in part c above and find the unknown value(be sure to show all of your work)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2


Chemistry, 23.06.2019 09:30
Where are the noble gases located in the periodic table? a. in the center b. on the left side c. in the upper right corner d. on the far right side
Answers: 1

Chemistry, 23.06.2019 16:30
If 950 joules of heat are needed to increase the temperature of 45.6 grams of an unknown metal from 25.0 celsius to 90.0 celsius, what is the specific heat capacity of the metal in j/g celsius
Answers: 2
You know the right answer?
Questions

Mathematics, 20.01.2021 20:50


Chemistry, 20.01.2021 20:50

History, 20.01.2021 20:50

Chemistry, 20.01.2021 20:50




Mathematics, 20.01.2021 20:50


English, 20.01.2021 20:50

History, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50





Mathematics, 20.01.2021 20:50

English, 20.01.2021 20:50