
Chemistry, 06.12.2019 05:31 taylortayshaun7
The radioactive decay of a certain sample produced 846 disintegrations per minute. exactly 3.00 days later, the rate of decay was found to be 269 disintegrations per minute. calculate the half-life, in days, for the decay of this sample.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3
You know the right answer?
The radioactive decay of a certain sample produced 846 disintegrations per minute. exactly 3.00 days...
Questions

Mathematics, 23.10.2020 20:20




Biology, 23.10.2020 20:20



Mathematics, 23.10.2020 20:20


Chemistry, 23.10.2020 20:20

English, 23.10.2020 20:20


Mathematics, 23.10.2020 20:20

English, 23.10.2020 20:20

Mathematics, 23.10.2020 20:20

Mathematics, 23.10.2020 20:20

English, 23.10.2020 20:20


Chemistry, 23.10.2020 20:20

Arts, 23.10.2020 20:20

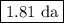
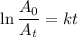
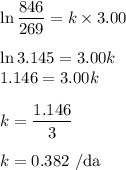
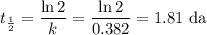
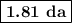 .
.

