
Chemistry, 16.10.2019 06:30 wafflewarriormg
Given the following balanced chemical reaction, what volume of 3.0m h2so4 is required to neutralize 60.0 ml of 0.5m naoh? be sure to include the formula and show your work for each step in the calculation.


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Given the following balanced chemical reaction, what volume of 3.0m h2so4 is required to neutralize...
Questions


History, 30.10.2019 07:31


Mathematics, 30.10.2019 07:31


Mathematics, 30.10.2019 07:31



Mathematics, 30.10.2019 07:31

History, 30.10.2019 07:31


Mathematics, 30.10.2019 07:31



Mathematics, 30.10.2019 07:31

Mathematics, 30.10.2019 07:31

Mathematics, 30.10.2019 07:31

English, 30.10.2019 07:31


Mathematics, 30.10.2019 07:31

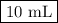

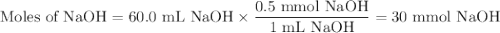
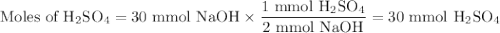
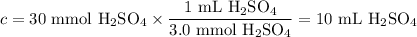
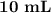 H₂SO₄.
H₂SO₄.

