
Shana solves stoichiometry problems using the equation for the synthesis of water. which interpretation of the balanced equation would cause shana to make a mistake? two moles of hydrogen react with one mole of oxygen to form two moles of water. two grams of hydrogen react with one gram of oxygen to form two grams of water. two molecules of hydrogen react with one molecule of oxygen to form two molecules of water. two liters of hydrogen react with one liter of oxygen to form two liters of water.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 06:30
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent.true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight.a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
Shana solves stoichiometry problems using the equation for the synthesis of water. which interpretat...
Questions

Mathematics, 21.01.2021 06:00

Mathematics, 21.01.2021 06:00


History, 21.01.2021 06:00

Mathematics, 21.01.2021 06:00

Social Studies, 21.01.2021 06:00



Social Studies, 21.01.2021 06:00


Mathematics, 21.01.2021 06:00




Mathematics, 21.01.2021 06:00

Health, 21.01.2021 06:00

Business, 21.01.2021 06:00

Social Studies, 21.01.2021 06:00


Mathematics, 21.01.2021 06:00

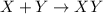
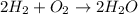
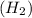 react with 1 mole of oxygen
react with 1 mole of oxygen 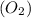 to form 2 moles of water
to form 2 moles of water 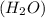 as a single product.
as a single product.

