
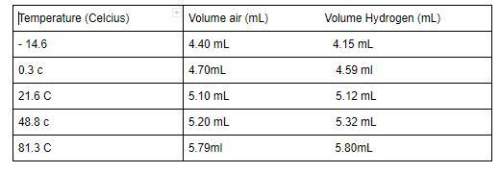
Temperature (celcius) volume air (ml) volume hydrogen (ml) - 14.6 4.40 ml 4.15 ml 0.3 c 4.70ml 4.59 ml 21.6 c 5.10 ml 5.12 ml 48.8 c 5.20 ml 5.32 ml 81.3 c 5.79ml 5.80ml data analysis: create a separate graph of temperature vs. volume for each of the gas samples. you are encouraged to use graphing software or online tools to create the graphs; be sure to take screenshots of the graphs that also include your data.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Temperature (celcius) volume air (ml) volume hydrogen (ml) - 14.6 4.40 ml 4.15 ml 0.3 c 4.70ml 4.59...
Questions


Mathematics, 15.04.2021 20:50

Physics, 15.04.2021 20:50

Mathematics, 15.04.2021 20:50





Mathematics, 15.04.2021 21:00

Chemistry, 15.04.2021 21:00

Mathematics, 15.04.2021 21:00

Mathematics, 15.04.2021 21:00


Biology, 15.04.2021 21:00

Mathematics, 15.04.2021 21:00


Chemistry, 15.04.2021 21:00

History, 15.04.2021 21:00


Mathematics, 15.04.2021 21:00



