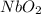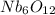Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
An oxide of niobium has a cubic unit cell in which there are oxide ions at the middle of each edge a...
Questions

Social Studies, 24.06.2019 05:00

Mathematics, 24.06.2019 05:00

Mathematics, 24.06.2019 05:00

Chemistry, 24.06.2019 05:00




Advanced Placement (AP), 24.06.2019 05:00

English, 24.06.2019 05:00



Computers and Technology, 24.06.2019 05:00


Geography, 24.06.2019 05:00

Mathematics, 24.06.2019 05:00


Mathematics, 24.06.2019 05:00


Mathematics, 24.06.2019 05:00

Mathematics, 24.06.2019 05:00