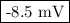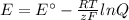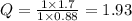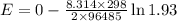Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
What would the potential of a standard hydrogen electrode (s. h.e.) be if it was under the following...
Questions


Mathematics, 23.04.2021 18:10


Mathematics, 23.04.2021 18:10

Physics, 23.04.2021 18:10

Mathematics, 23.04.2021 18:10

Mathematics, 23.04.2021 18:10


Mathematics, 23.04.2021 18:20

Spanish, 23.04.2021 18:20







Mathematics, 23.04.2021 18:20

Law, 23.04.2021 18:20

Mathematics, 23.04.2021 18:20

Mathematics, 23.04.2021 18:20