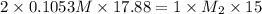Chemistry, 25.11.2019 23:31 josephsky420
A15.00-ml sample of a naoh solution of unknown concentration requires 17.88 ml of a 0.1053 m h2so4 solution to reach the equivalence point in a titration. what is the concentration of the naoh solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

You know the right answer?
A15.00-ml sample of a naoh solution of unknown concentration requires 17.88 ml of a 0.1053 m h2so4 s...
Questions

Mathematics, 24.10.2020 01:40

Mathematics, 24.10.2020 01:40

Mathematics, 24.10.2020 01:40

English, 24.10.2020 01:40

Mathematics, 24.10.2020 01:40


English, 24.10.2020 01:40

Mathematics, 24.10.2020 01:40

English, 24.10.2020 01:40

Mathematics, 24.10.2020 01:40



Mathematics, 24.10.2020 01:40

Mathematics, 24.10.2020 01:40


Mathematics, 24.10.2020 01:40



Mathematics, 24.10.2020 01:40

Mathematics, 24.10.2020 01:40

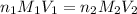
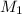 = molarity of
= molarity of 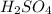 solution = 0.1053 M
solution = 0.1053 M
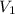 = volume of
= volume of 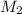 = molarity of
= molarity of 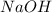 solution = 0.46 M
solution = 0.46 M
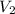 = volume of
= volume of 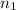 = valency of
= valency of 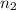 = valency of
= valency of