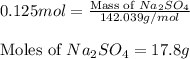The following reaction shows sodium hydroxide reacting with sulfuric acid.
naoh + h2so4...

Chemistry, 30.01.2020 17:02 xXFLUFFYXx
The following reaction shows sodium hydroxide reacting with sulfuric acid.
naoh + h2so4 → na2so4 + h2o
how many grams of na2so4 are produced from 10.0 grams of naoh?
(molar mass of na = 22.989 g/mol, o = 15.999 g/mol, h = 1.008 g/mol, s = 32.065 g/mol)
17.8 grams
19.2 grams
35.5 grams
38.5 grams

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 07:00
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1

Chemistry, 23.06.2019 15:30
Express your answer using two significant figures. 1.7 km^2
Answers: 2
You know the right answer?
Questions



Chemistry, 31.01.2020 14:52



History, 31.01.2020 14:52



Mathematics, 31.01.2020 14:52




Mathematics, 31.01.2020 14:52




Geography, 31.01.2020 14:52

History, 31.01.2020 14:52

Chemistry, 31.01.2020 14:52

History, 31.01.2020 14:52

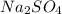 produced will be 17.8 grams.
produced will be 17.8 grams. 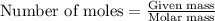 ....(1)
....(1)![NaOH=[(1\times 22.989)+(1\times 15.999)+(1\times 1.008)]g/mol=39.996g/mol](/tpl/images/0486/6119/380bc.png)
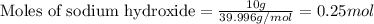
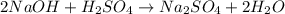
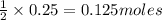 of sodium sulfate.
of sodium sulfate.![Na_2SO_4=[(2\times 22.989)+(1\times 32.065)+(4\times 15.999)]g/mol=142.039g/mol](/tpl/images/0486/6119/878b0.png)