
Chemistry, 28.12.2019 23:31 tagerryawilson6
During a laboratory experiment, a 3.24-gram sample of nahco3 was thermally decomposed. in this experiment, carbon dioxide and water vapors escape and are combined to form carbonic acid. after decomposition, the sample weighed 2.19 grams. calculate the percentage yield of carbonic acid for the reaction. describe the calculation process in detail. nahco3 → na2co3 + h2co3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
During a laboratory experiment, a 3.24-gram sample of nahco3 was thermally decomposed. in this exper...
Questions




Biology, 02.09.2019 09:00



Mathematics, 02.09.2019 09:00


Mathematics, 02.09.2019 09:00

History, 02.09.2019 09:00



History, 02.09.2019 09:00

Chemistry, 02.09.2019 09:00

Spanish, 02.09.2019 09:00


History, 02.09.2019 09:00

Mathematics, 02.09.2019 09:00

Mathematics, 02.09.2019 09:00

Chemistry, 02.09.2019 09:00

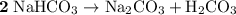 .
.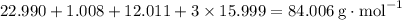 .H₂CO₃:
.H₂CO₃: 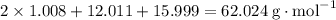 .
.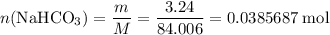 .
.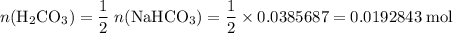
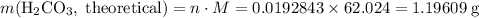 .
.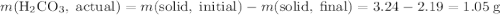 .
. .
.

