have the same molecular weight

Chemistry, 28.09.2019 00:50 gcristhian8863
Amole of the element u (uranium) and a mole of co2:
have the same molecular weight
have the same atomic mass
have an equal number of particles
have an equal number of atoms

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
Amole of the element u (uranium) and a mole of co2:
have the same molecular weight
have the same molecular weight
Questions

Biology, 22.09.2019 21:20


Mathematics, 22.09.2019 21:20

Mathematics, 22.09.2019 21:20






English, 22.09.2019 21:20

Chemistry, 22.09.2019 21:20

Biology, 22.09.2019 21:20






Mathematics, 22.09.2019 21:20

Mathematics, 22.09.2019 21:20

Mathematics, 22.09.2019 21:20

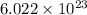 number of particles.
number of particles.

