

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
The reaction a + 2b + c -- > d + 2e is first order in reactant a, first order in b, and second or...
Questions

Mathematics, 05.05.2020 14:43

Mathematics, 05.05.2020 14:43

Mathematics, 05.05.2020 14:43




Mathematics, 05.05.2020 14:43

English, 05.05.2020 14:43









English, 05.05.2020 14:43

Biology, 05.05.2020 14:43


Social Studies, 05.05.2020 14:43

![\text{Rate}=k[A][B][C]^2](/tpl/images/0287/3858/2c5a0.png)
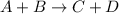
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0287/3858/10aeb.png)
![[A]](/tpl/images/0287/3858/6aa06.png) and
and ![[B]](/tpl/images/0287/3858/db909.png) = concentration of A and B reactant
= concentration of A and B reactant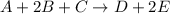
![\text{Rate}=k[A]^1[B]^1[C]^2](/tpl/images/0287/3858/d33f5.png)


