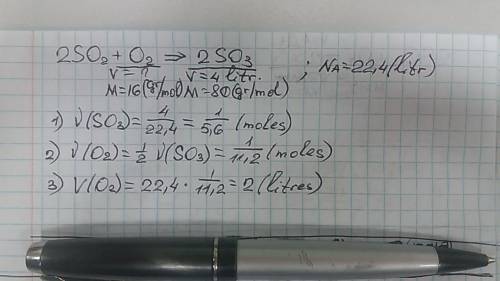Chemistry, 19.10.2019 10:10 Callmetay24
In the reaction 2so2(g) + o2(g) -> 2so3(g) how many liters of oxygen are needed produced 4 liters of so3 at stp?
need this asap

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1

Chemistry, 23.06.2019 06:40
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1
You know the right answer?
In the reaction 2so2(g) + o2(g) -> 2so3(g) how many liters of oxygen are needed produced 4 liter...
Questions



Mathematics, 20.08.2021 21:10





Mathematics, 20.08.2021 21:10

English, 20.08.2021 21:10

Computers and Technology, 20.08.2021 21:10

Mathematics, 20.08.2021 21:10


Mathematics, 20.08.2021 21:10



History, 20.08.2021 21:10


History, 20.08.2021 21:10

Mathematics, 20.08.2021 21:10