
Chemistry, 23.09.2019 15:30 babyrocks7300
If 30.0 ml of ca(oh)2 with an unknown concentration is neutralized by 40.0 ml of 0.175 m hcl, what is the concentration of the ca(oh)2 solution? show all of the work needed to solve this problem. (2 points) ca(oh)2 + 2hcl yields 2h2 o + cacl2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
An example of technology is the a. addition of a side group to an organic molecule during synthesis. b. use of a new antibiotic to fight an infection. c. measurement of iron concentration in a water sample. d. study of atomic fusion reactions
Answers: 3

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

You know the right answer?
If 30.0 ml of ca(oh)2 with an unknown concentration is neutralized by 40.0 ml of 0.175 m hcl, what i...
Questions


Mathematics, 02.12.2021 09:10

English, 02.12.2021 09:10

Mathematics, 02.12.2021 09:10

Mathematics, 02.12.2021 09:10

Advanced Placement (AP), 02.12.2021 09:10

English, 02.12.2021 09:10


English, 02.12.2021 09:10

Mathematics, 02.12.2021 09:20





English, 02.12.2021 09:20

Mathematics, 02.12.2021 09:20



Biology, 02.12.2021 09:20

Mathematics, 02.12.2021 09:20

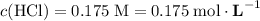 .
.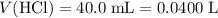 .
.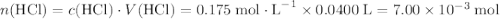 .
.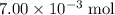 of HCl will neutralize only half that much Ca(OH)₂.
of HCl will neutralize only half that much Ca(OH)₂.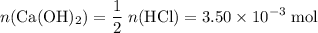 .
.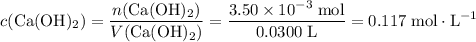 .
.

