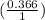Chemistry, 09.11.2019 05:31 barloase5747
What would the initial ph of a carbonic acid - bicarbonate (ka=4.27 x 10-7) buffer be if the ratio of conjugate base to acid is 0.366 to 1?
a.
1.67
b.
1.81
c.
5.79
d.
5.85
e.
5.93

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
What would the initial ph of a carbonic acid - bicarbonate (ka=4.27 x 10-7) buffer be if the ratio o...
Questions


Mathematics, 09.11.2019 04:31

Health, 09.11.2019 04:31


History, 09.11.2019 04:31





Geography, 09.11.2019 04:31

History, 09.11.2019 04:31


Mathematics, 09.11.2019 04:31


Mathematics, 09.11.2019 04:31




Mathematics, 09.11.2019 05:31

![(\frac{[A^-]}{[HA]})](/tpl/images/0366/7089/ee08c.png) , where A^- is the concentration of the conjugate base of the weak acid & HA is the concentration of the acid.
, where A^- is the concentration of the conjugate base of the weak acid & HA is the concentration of the acid.