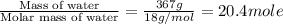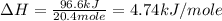Chemistry, 28.09.2019 22:00 emalvidrez5205
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes place in the calorimeter, and the temperature rises to 87°c. the calorimeter contains 367 g of water, which has a specific heat of 4.18 j/(g·°c). calculate the enthalpy change (δh) during this reaction

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes pl...
Questions

English, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31






Mathematics, 05.11.2019 08:31

English, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31

Social Studies, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31

Biology, 05.11.2019 08:31




History, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31

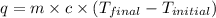
 = specific heat of water =
= specific heat of water = 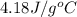
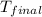 = final temperature of water =
= final temperature of water = 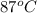
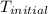 = initial temperature of metal =
= initial temperature of metal = 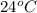
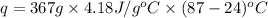
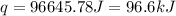
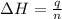
 = enthalpy change = ?
= enthalpy change = ?