Read the given equation. 2na + 2h2o → 2naoh + h2
during a laboratory experiment, a certain qu...

Chemistry, 03.11.2019 07:31 twalters88
Read the given equation. 2na + 2h2o → 2naoh + h2
during a laboratory experiment, a certain quantity of sodium metal reacted with water to produce sodium hydroxide and hydrogen gas. what was the initial quantity of sodium metal used if 6.30 liters of h2 gas were produced at stp?
a.) 10.3 grams
b.) 12.9 grams
c.) 14.7 grams
d.) 15.2 grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
Questions


Mathematics, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00

Health, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00



Mathematics, 13.07.2019 12:00

Social Studies, 13.07.2019 12:00


Mathematics, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00


History, 13.07.2019 12:00

Biology, 13.07.2019 12:00

Biology, 13.07.2019 12:00

Computers and Technology, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00

Biology, 13.07.2019 12:00

History, 13.07.2019 12:00

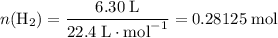 .
.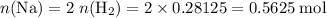 .
.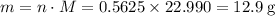 .
.

