
Chemistry, 06.10.2019 16:30 4presidents
Questions 3-6 refer to rhe solutions below:
(a)100ml of 1.0 m ch3cooh mixed with 100ml of 1.0 m ch3coona
(b)100ml of 1.0 m hbr mixed with 100ml of 1.0 m kbr
(c)100ml of 1.0 m hi mixed with 100ml of 1.0 m naoh
(d)100ml of 1.0 m nh4cl mixed with 100ml of 1.0 m nh3
(e)100ml of 1.0 m naoh mixed with 100ml of 1.0 m ch3cooh
3.) which solution will produce a buffer with a ph < 6.5
4.) which solution will produce a buffer with a ph > 7.5
5.) which solutiom will have a ph of about 7
6.) which solution will be the most acidic

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Questions 3-6 refer to rhe solutions below:
(a)100ml of 1.0 m ch3cooh mixed with 100ml of 1.0...
(a)100ml of 1.0 m ch3cooh mixed with 100ml of 1.0...
Questions


Biology, 23.09.2019 11:10

Geography, 23.09.2019 11:10

English, 23.09.2019 11:10

History, 23.09.2019 11:10

Mathematics, 23.09.2019 11:10


Mathematics, 23.09.2019 11:10

Business, 23.09.2019 11:10



Geography, 23.09.2019 11:20


Mathematics, 23.09.2019 11:20


English, 23.09.2019 11:20



Geography, 23.09.2019 11:20

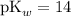 :
:![\displaystyle \text{pH} = \text{pK}_a + \log{\frac{[\text{Conjugate Ion}]}{[\text{Weak Acid}]}}](/tpl/images/0294/2613/aa9f4.png) .
.![\displaystyle \log{\frac{[\text{Conjugate Ion}]}{[\text{Weak Acid}]}} =\ln{1} = 0](/tpl/images/0294/2613/7ba22.png) .
. ![\displaystyle [\text{H}^{+}] = [\text{HBr}] \\\phantom{[\text{H}^{+}]}= \frac{n}{V} \\\phantom{[\text{H}^{+}]}= \frac{c(\text{HBr})\cdot V(\text{HBr})}{V(\text{HBr})+V(\text{KBr})}\\\phantom{[\text{H}^{+}]}=\frac{0.100\;\text{L}\times 1.0\;\text{mol}\cdot\text{L}^{-1}}{0.100\;\text{L}+0.100\;\text{L}} \\\phantom{[\text{H}^{+}]}= 0.50\;\text{mol}\cdot\text{L}^{-1}](/tpl/images/0294/2613/25b47.png) .
.![\text{pH} = -\log{[\text{H}^{+}] = -\log{0.50} \approx {\bf 0.30}](/tpl/images/0294/2613/3101f.png) .
.![\displaystyle \textbf{pOH} = \text{pK}_b + \log{\frac{[\text{Conjugate Ion}]}{[\text{Weak Base}]}}= 4.75 + \log{1} = 4.75](/tpl/images/0294/2613/116dc.png) .
.![n(\text{CH}_3\text{COO}^{-}) = n(\text{NaOH}] = c\cdot V = 0.10\;\text{mol}](/tpl/images/0294/2613/0dd47.png) .
.![\displaystyle [\text{CH}_3\text{COO}^{-}] = \frac{n}{V} = {0.10}{0.10 + 0.10} = 0.50 \;\text{mol}\cdot\text{L}^{-1}](/tpl/images/0294/2613/d5eca.png) .
.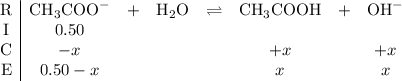
 will be much smaller than
will be much smaller than 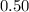 such that
such that 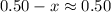 .
.![\displaystyle \frac{[\text{CH}_3\text{COOH}]\cdot[\text{OH}^{-}]}{[\text{CH}_3\text{COO}^{-}]} = \text{K}_b(\text{CH}_3\text{COO}^{-}) \\\phantom{\displaystyle \frac{[\text{CH}_3\text{COOH}]\cdot[\text{OH}^{-}]}{[\text{CH}_3\text{COO}^{-}]} }= \frac{\text{K}_w}{\text{K}_a(\text{CH}_3\text{COOH})} \\\phantom{\displaystyle \frac{[\text{CH}_3\text{COOH}]\cdot[\text{OH}^{-}]}{[\text{CH}_3\text{COO}^{-}]}} = \frac{10^{-14}}{1.75\times 10^{-5}} = 5.71\times 10^{-10}](/tpl/images/0294/2613/44f5d.png) .
.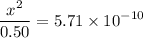 .
.![[\text{OH}^{-}] = x \approx 1.69\times 10^{-5}\;\text{mol}\cdot\text{L}^{-1}](/tpl/images/0294/2613/ed038.png) .
.![\text{pH} = \text{pK}_w + \log{[\text{OH}^{-}]} = 9.23](/tpl/images/0294/2613/43c0c.png) .
.


