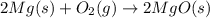Chemistry, 20.11.2019 16:31 reganleigh00
The oxidation of magnesium to form magnesium oxide is shown by which balanced chemical equation?
a) mg(s) + o2(g) → mgo(s)
b) 2mg(s) + o2(g) → 2mgo(s)
c) 2mgo(s) → 2mg(s) + o2(g)
d) 2mg(s) → 2mgo(s) + o2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
The oxidation of magnesium to form magnesium oxide is shown by which balanced chemical equation?
Questions






Social Studies, 28.02.2020 21:17




English, 28.02.2020 21:17





Mathematics, 28.02.2020 21:17


Mathematics, 28.02.2020 21:17



English, 28.02.2020 21:17