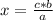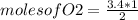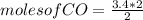Chemistry, 05.01.2020 02:31 katherineedwards1105
Study the reaction.
2co + o2 → 2co2
if 3.4 moles of carbon dioxide (co2) form at the end of the reaction, how many moles of carbon monoxide (co) and oxygen gas (o2) entered the reaction?
2.0 moles of carbon monoxide and 1.0 moles of oxygen gas
6.8 moles of carbon monoxide and 3.4 moles of oxygen gas
3.4 moles of carbon monoxide and 1.7 moles of oxygen gas

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Study the reaction.
2co + o2 → 2co2
if 3.4 moles of carbon dioxide (co2) for...
2co + o2 → 2co2
if 3.4 moles of carbon dioxide (co2) for...
Questions