
Chemistry, 05.01.2020 14:31 twirlergirl800
Achemistry student weighs out 0.0634g of formic acid hcho2 into a 250.ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1800m naoh solution. calculate the volume of naoh solution the student will need to add to reach the equivalence point. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
Achemistry student weighs out 0.0634g of formic acid hcho2 into a 250.ml volumetric flask and dilute...
Questions







Physics, 20.02.2020 16:27


Biology, 20.02.2020 16:31






History, 20.02.2020 16:45





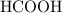 :
: .
.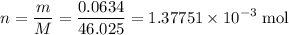 .
.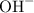 ion to neutralize each carbonyl group
ion to neutralize each carbonyl group 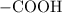 .
.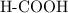 molecule. Each formula unit of NaOH supplies one
molecule. Each formula unit of NaOH supplies one  of formic acid in the volumetric flask. It will take the same number of NaOH formula units to reach the equivalence point.
of formic acid in the volumetric flask. It will take the same number of NaOH formula units to reach the equivalence point.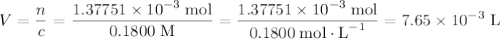 .
.

