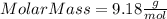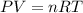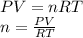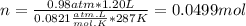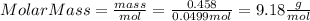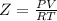Chemistry, 04.02.2020 01:53 sebastiantroysmith
Quickly !
part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1.20 l at 287 k and 0.980 atm. show your work. part 2. if this sample was placed under extreme pressure, describe how the actual volume would compare to the predicted volume. explain your answer.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

You know the right answer?
Quickly !
part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1...
part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1...
Questions

Mathematics, 01.03.2021 17:10

Mathematics, 01.03.2021 17:10


Mathematics, 01.03.2021 17:10

Mathematics, 01.03.2021 17:10

History, 01.03.2021 17:10



English, 01.03.2021 17:10

Mathematics, 01.03.2021 17:10

Mathematics, 01.03.2021 17:10


Spanish, 01.03.2021 17:10

History, 01.03.2021 17:10

Mathematics, 01.03.2021 17:10

Mathematics, 01.03.2021 17:20