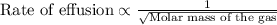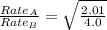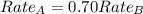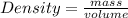Chemistry, 29.09.2019 01:30 zmoore3793
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. molar mass comparison gas molar mass a 4.00 g/mol b 2.01 g/mol which statement describes the density and effusion of both gases at stp? gas a has a higher density and effuses faster than gas b. gas a has a higher density and effuses slower than gas b. gas a has a lower density and effuses faster than gas b. gas a has a lower density and effuses slower than gas b.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3


Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. mola...
Questions




World Languages, 01.10.2019 07:20

World Languages, 01.10.2019 07:20

Mathematics, 01.10.2019 07:20




English, 01.10.2019 07:20



Mathematics, 01.10.2019 07:20

Physics, 01.10.2019 07:20


Mathematics, 01.10.2019 07:20

Mathematics, 01.10.2019 07:20

Mathematics, 01.10.2019 07:20

History, 01.10.2019 07:20

Chemistry, 01.10.2019 07:20