
Chemistry, 01.02.2020 13:42 dianaparra826
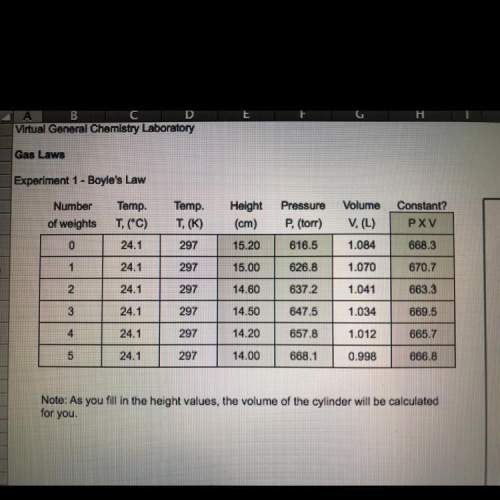
Derive the relationship between v1 and v2, the volumes of a gas at two pressures, p1 and p2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
Derive the relationship between v1 and v2, the volumes of a gas at two pressures, p1 and p2
...
...
Questions

English, 22.08.2019 05:30






Mathematics, 22.08.2019 05:30



Biology, 22.08.2019 05:30

Social Studies, 22.08.2019 05:30

Mathematics, 22.08.2019 05:30


Mathematics, 22.08.2019 05:30

Mathematics, 22.08.2019 05:30

Mathematics, 22.08.2019 05:30

Geography, 22.08.2019 05:30


History, 22.08.2019 05:30



