
Chemistry, 02.10.2019 01:00 divagothboi
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statements is correct about the δh for the products?
a. it is less than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
b. it is less than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.
c. it is greater than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
d. it is greater than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statement...
Questions

English, 12.05.2020 04:57

History, 12.05.2020 04:57

Mathematics, 12.05.2020 04:57



Mathematics, 12.05.2020 04:57






Biology, 12.05.2020 04:57




Mathematics, 12.05.2020 04:57


Mathematics, 12.05.2020 04:57

Spanish, 12.05.2020 04:57

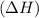 comes out to be positive.
comes out to be positive.

