
Chemistry, 22.11.2019 06:31 myaaa13754
Find the concentration of h+ ions at a ph = 11 and
ph = 6. then divide the concentration of h+ ions at a
ph = 11 by the of h+ ions at a ph = 6. record your answer in table c.
what is the concentration of h+ ions at a ph = 11?
mol/l
what is the concentration of h+ ions at a ph = 6?
mol/l
how many fewer h+ ions are there in a solution at a
ph = 11 than in a solution at a ph = 6?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
Find the concentration of h+ ions at a ph = 11 and
ph = 6. then divide the concentration of h+...
ph = 6. then divide the concentration of h+...
Questions










Mathematics, 30.06.2019 06:10





Mathematics, 30.06.2019 06:10

Mathematics, 30.06.2019 06:10


History, 30.06.2019 06:10


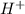 ion concentration.
ion concentration.![pH=-\log[H^+]](/tpl/images/0386/0640/cf945.png)
![11=-\log[H^+]](/tpl/images/0386/0640/c91a3.png)
![[H^+]=1\times 10^{-11} mol/L](/tpl/images/0386/0640/23467.png) ..(1)
..(1)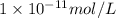 .
.![6=-\log[H^+]'](/tpl/images/0386/0640/502fa.png)
![[H^+]'=1\times 10^{-6} mol/L](/tpl/images/0386/0640/fdc73.png) ..(2)
..(2)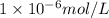 .
.![\frac{[H^+]}{[H^+]'}=\frac{1\times 10^{-11} mol/L}{1\times 10^{-6} mol/L}=1\times 10^{-5}](/tpl/images/0386/0640/2e79f.png)
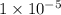 .
.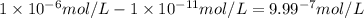
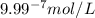 ions fewer than in a solution at a pH = 6
ions fewer than in a solution at a pH = 6


