
Chemistry, 24.11.2019 10:31 BreBreDoeCCx
Must show work for questions 5 and 6. ( also explain, i don't understand)
4. consider the reaction. which of the following is true?
5. what is the specific heat of hg if it requires 166.7 j to change the temperature of 15.0 g mercury from 25.00°c to 33.00°c?
6. consider the reaction. when a 17.2-g sample of ethyl alcohol (molar mass = 46.1 g/mol) is burned, how much energy is released as heat?
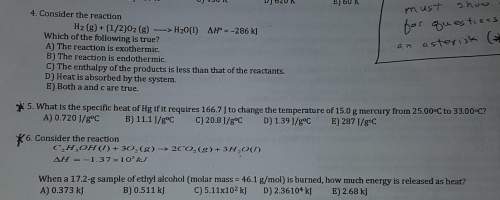

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1


Chemistry, 23.06.2019 15:30
Ms. sullivan's class is designing a miniature roller coaster. after first setting it up, they find that it didn't have enough speed to go through the loop de loop. instead of taking out the loop, they decide to increase the height of the first hill the coaster goes down, and this adds the speed needed to make it through the loop. what would be a drawback to this plan in real life? a) no one likes loop de loops. b) there is no downside to this plan. c) a higher hill means a scarier ride. d) a higher hill takes more time and material to build.
Answers: 1
You know the right answer?
Must show work for questions 5 and 6. ( also explain, i don't understand)
4. consider the...
4. consider the...
Questions


English, 17.04.2021 19:40

Arts, 17.04.2021 19:40

Computers and Technology, 17.04.2021 19:40


Chemistry, 17.04.2021 19:40

English, 17.04.2021 19:40



History, 17.04.2021 19:40


Social Studies, 17.04.2021 19:40

Social Studies, 17.04.2021 19:40



Mathematics, 17.04.2021 19:40

History, 17.04.2021 19:40


English, 17.04.2021 19:40




