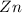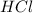When zinc reacts with hydrochloric acid, it produces hydrogen gas. as the reaction proceeds, why does the rate of production of hydrogen gas decrease? the concentration of hydrogen gas decreases. the concentration of the reactants decreases. the hydrogen gas formed inhibits the reaction. the hydrogen gas also reacts with the zinc metal.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
When zinc reacts with hydrochloric acid, it produces hydrogen gas. as the reaction proceeds, why doe...
Questions



Biology, 19.11.2020 01:40

Mathematics, 19.11.2020 01:40

English, 19.11.2020 01:40

Mathematics, 19.11.2020 01:40

Mathematics, 19.11.2020 01:40

Mathematics, 19.11.2020 01:50

Mathematics, 19.11.2020 01:50


Mathematics, 19.11.2020 01:50


Social Studies, 19.11.2020 01:50


Spanish, 19.11.2020 01:50




History, 19.11.2020 01:50

Health, 19.11.2020 01:50

![Rate=k[A]^x[B]^y](/tpl/images/0383/1895/ddde1.png)