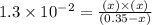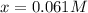Chemistry, 03.02.2020 23:00 MARTINASLACK299
What are the concentrations of hso4−, so42-, and h+ in a 0.35 m khso4 solution? (hint: h2so4 is a strong acid; ka for hso4− = 1.3 ✕ 10−

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

You know the right answer?
What are the concentrations of hso4−, so42-, and h+ in a 0.35 m khso4 solution? (hint: h2so4 is a...
Questions




Biology, 13.07.2019 05:30


Mathematics, 13.07.2019 05:30

History, 13.07.2019 05:30


Mathematics, 13.07.2019 05:30


Mathematics, 13.07.2019 05:30

Mathematics, 13.07.2019 05:30


Mathematics, 13.07.2019 05:30



Computers and Technology, 13.07.2019 05:30

Mathematics, 13.07.2019 05:30

Biology, 13.07.2019 05:30

Engineering, 13.07.2019 05:30

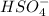 ,
, 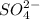 and
and 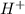 are 0.29 M, 0.061 M and 0.061 M respectively.
are 0.29 M, 0.061 M and 0.061 M respectively.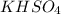 is:
is: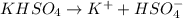
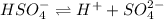
![K_a=\frac{[H^+][SO_4^{2-}]}{[HSO_4^-]}](/tpl/images/0498/1651/2180b.png)