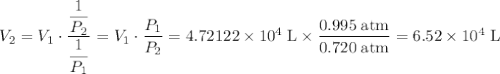Chemistry, 13.10.2019 15:50 AphEngland
Aweather balloon is filled with helium that occupies a volume of 5.57 104 l at 0.995 atm and 32.0°c. after it is released, it rises to a location where the pressure is 0.720 atm and the temperature is -14.5°c. what is the volume of the balloon at that new location?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 09:00
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2
You know the right answer?
Aweather balloon is filled with helium that occupies a volume of 5.57 104 l at 0.995 atm and 32.0°c....
Questions




Arts, 01.04.2020 20:23

Physics, 01.04.2020 20:23

English, 01.04.2020 20:23


English, 01.04.2020 20:23

Mathematics, 01.04.2020 20:23


Mathematics, 01.04.2020 20:23


Mathematics, 01.04.2020 20:23


English, 01.04.2020 20:23



History, 01.04.2020 20:23

Mathematics, 01.04.2020 20:23

Mathematics, 01.04.2020 20:23

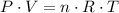 ,
,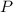 is the pressure of the gas,
is the pressure of the gas,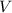 is the volume of the gas,
is the volume of the gas, 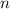 is the number of gas particles in the gas,
is the number of gas particles in the gas, 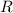 is the ideal gas constant, and
is the ideal gas constant, and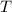 is the absolute temperature of the gas in degrees Kelvins.
is the absolute temperature of the gas in degrees Kelvins.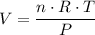 .
. 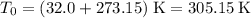 ,
,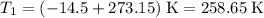 .
.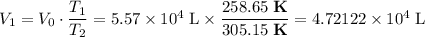 .
.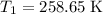 and change the pressure on the gas:
and change the pressure on the gas: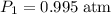 ,
,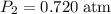 .
.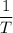 if both
if both