
Chemistry, 13.11.2019 00:31 zedanabdul198
Which statement can best be concluded from the ideal gas law? the product of pressure and volume of an ideal gas is proportional to the absolute temperature. all collisions between atoms or molecules are perfectly elastic and are not the result of any attractive forces. the temperature, pressure, and volume of a gas are all related. the behavior of a gas under real conditions does not obey the ideal gas law.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1


Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
Which statement can best be concluded from the ideal gas law? the product of pressure and volume of...
Questions

Mathematics, 07.12.2020 22:20

Mathematics, 07.12.2020 22:20



Mathematics, 07.12.2020 22:20

Health, 07.12.2020 22:20




Social Studies, 07.12.2020 22:20






Biology, 07.12.2020 22:20


SAT, 07.12.2020 22:20


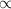 nT
nT

