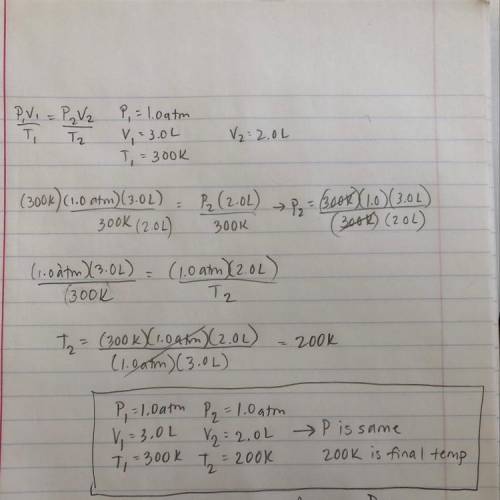Chemistry, 14.10.2019 21:30 gajdmaciej9502
Need asap
the volume of a gas is 3.0 l, the pressure is 1.0 atm, and the temperature is 300 k. a chemist changes one factor while keeping another constant so that the new volume is 2.0 l. which of the following could be the new conditions?
a)the final pressure is 0.5 atm, while temperature is kept constant.
b)the final temperature is 200 k, while pressure remains constant.
c)the final pressure is 0.2 atm, while temperature is kept constant.
d)the final temperature is 350 k, while pressure remains constant.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
Need asap
the volume of a gas is 3.0 l, the pressure is 1.0 atm, and the temperature is 300 k...
the volume of a gas is 3.0 l, the pressure is 1.0 atm, and the temperature is 300 k...
Questions

Health, 06.05.2020 04:10

Computers and Technology, 06.05.2020 04:10



Mathematics, 06.05.2020 04:10

Mathematics, 06.05.2020 04:10

Chemistry, 06.05.2020 04:10




Mathematics, 06.05.2020 04:10







History, 06.05.2020 04:10

Computers and Technology, 06.05.2020 04:10