
Chemistry, 07.12.2019 17:31 xXCoryxKenshinXx
Calculate the mass of chromium metal produced when 425.0ml of 0.25m chromium(ll) nitrate reacts with a strip of zinc that remains in excess. also write and balance an equation.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
Calculate the mass of chromium metal produced when 425.0ml of 0.25m chromium(ll) nitrate reacts with...
Questions

History, 14.07.2019 19:20

English, 14.07.2019 19:20

History, 14.07.2019 19:20

Mathematics, 14.07.2019 19:20

History, 14.07.2019 19:20


Mathematics, 14.07.2019 19:20


History, 14.07.2019 19:20

History, 14.07.2019 19:20



Advanced Placement (AP), 14.07.2019 19:20

Biology, 14.07.2019 19:20


Advanced Placement (AP), 14.07.2019 19:20



History, 14.07.2019 19:20

Advanced Placement (AP), 14.07.2019 19:20

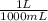 x
x 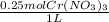 x
x 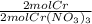 x
x 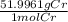 = 5.52 g
= 5.52 g 

